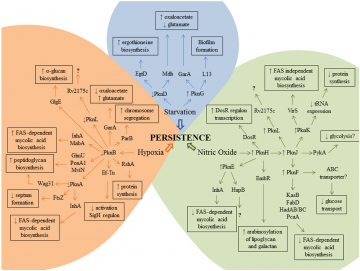
Persistence-associated STPK cell signalling network. Courtesy of Melissa Richard-Greenblatt.
The pathogenic success of Mtb is largely dependent on its ability to sense and adapt to the dynamic environment of the host. As such, Mtb employs an extensive signaling network of kinases and phosphatases involved in reversible protein phosphorylation.
During the pre-genomic era, we discovered the family of serine/threonine protein kinases (STPKs) in Mtb, and subsequently, our lab has been instrumental in elucidating functions of many of these STPKs over the past two decades. We have shown that these proteins control key aspects of Mtb physiology including virulence, dormancy, amino acid metabolism, cell wall biosynthesis and aspects of antibiotic resistance in response to external stimuli such as nitric oxide and antibiotics.
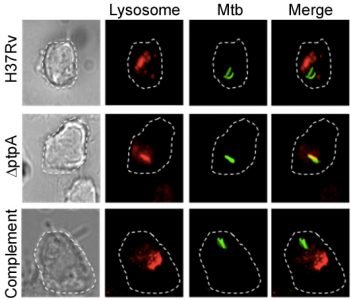
PtpA inhibition of lysosome fusion. Cred: Horacio Bach.
Work with a protein tyrosine phosphatase, PtpA, led us to the discovery of a key mechanism by which Mtb interferes with and subverts critical host defense mechanisms during Mtb infection. Mtb infects macrophages, the front-line defense against microbial invasion. Macrophages are typically able to destroy invading microorganisms by engulfing them into phagosomes which are then processed to fuse with lysosomes containing highly oxidative intermediates and hydrolytic enzymes. Mature phago-lysosomes are also markedly acidic through the action of vacuolar H+-ATPase (V-ATPase) pumps, making them potent microbicidal organelles.
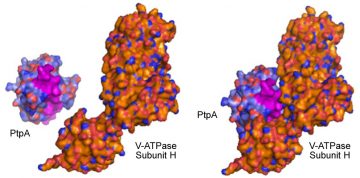
PtpA binding to V-ATPase Subunit H. Courtesy of Dennis Wong.
We found that Mtb secretes PtpA, whose substrate is a human macrophage protein, VPS33B, involved in membrane trafficking. The enzymatic activity of PtpA on VPS33B causes inhibition of phagosome-lysosome fusion, the hallmark of macrophage innate immune activity. In a follow up study, we showed that PtpA binds to the macrophage V-ATPase that drives luminal acidification. PtpA directly inhibits phagosome acidification, thus establishing its key role in Mtb survival and pathogenicity within host macrophages. This discovery provided the first Mtb protein that directly interferes with host functions and the mechanistic explanation to a key mycobacterial virulence trait discovered over 40 years ago.
The gene encoding PtpA is part of a three-gene operon and is flanked by ptkA and rv2235. We have shown that ptkA encodes an atypical but bona fide protein tyrosine kinase, the first of its kind identified in Mtb. We have also found that PtpA is subject to extensive post-translational modifications including phosphorylation by PtkA at two tyrosine residues; however, the exact role of this phosphorylation is not well defined. We are currently looking at the role of PtkA and its potential involvement in PtpA secretion, activation and other mycobacterial physiological processes.